Introduction to Electrochemistry
Electrochemistry - or more strictly electrosynthesis - is where the flow of electrons is used in place of a traditional chemical reagent to bring about a chemical reaction. Conceptually it is straightforward, two electrodes are placed within the reaction mixture to form part of the electrical circuit through which electrons flow (driven by an outside potential). At each electrode a reaction takes place, the reagent at the anode oxidises and releases electrons to the anode, and at the cathode the reagent is reduced by gaining electrons.
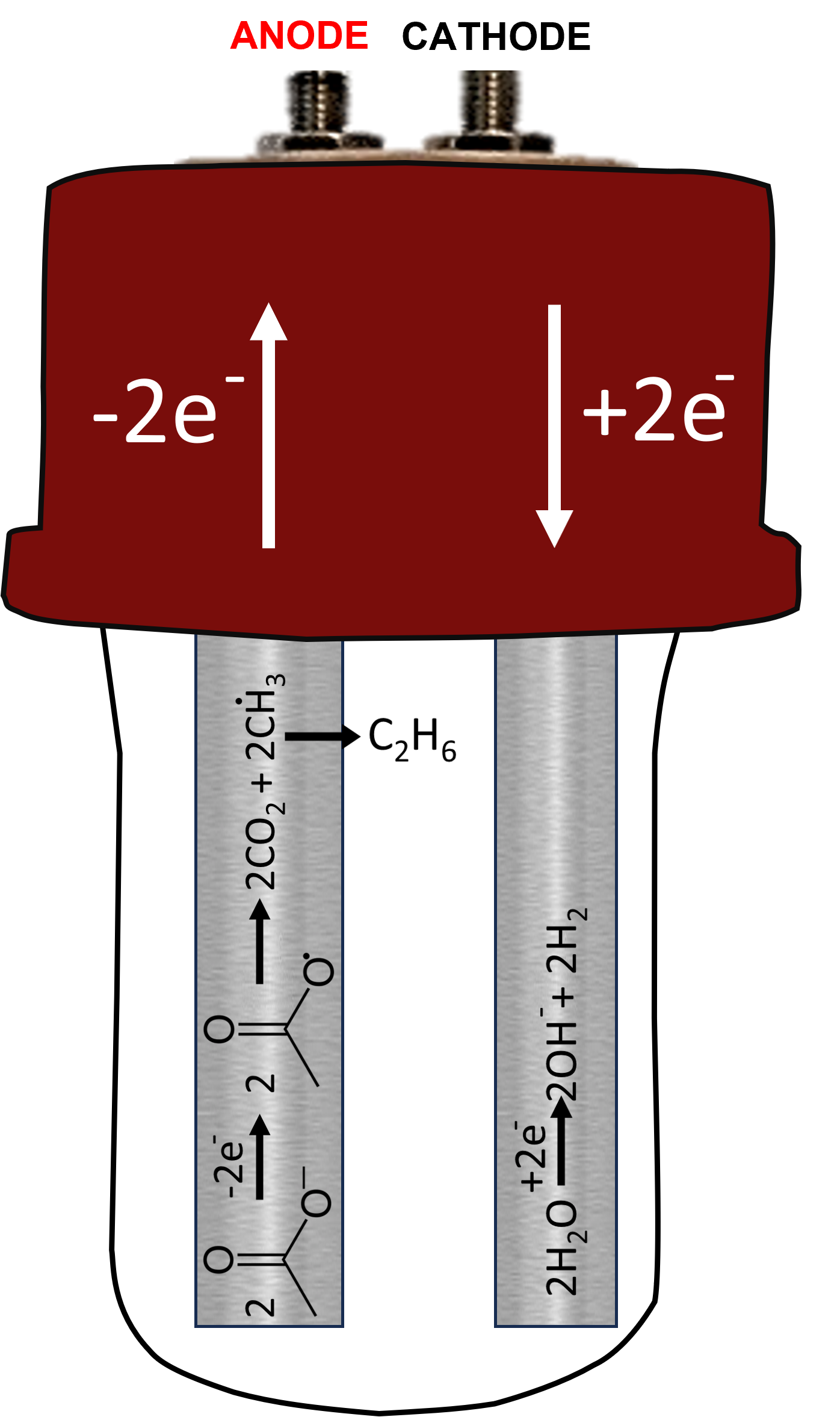
An early example is the Kolbe electrosynthesis from 1848 (we think Kolbe would have very much enjoyed the ElectroReact platform!). In water, the carboxylic acid deprotonates. Now at the anode, two carboxylate ions are oxidised, generating CO2 and forming identical radicals, which go on to react with each other to give a product. There are also two electrons liberated in this process, so for the overall reaction to balance there must be a second reaction at the cathode. This is usually H2 generation from H+, either from the original acid or from solvent. The energy for the reaction comes from the externally driven flow of electrons around the circuit. The reaction pathway is determined partly by the underlying chemistry and partly by the reactor characteristics - there is an intersection of chemistry and technology in determining the final outcome.
The choice of the reactor is important for the success of electrochemistry - a well characterised reactor capable of operating under inert conditions when required, and one that allows easy sampling or addition or material. Most importantly, a reactor should control key factors such as electrode spacing and mixing to enable the most reproducible science. This is why we designed ElectroReact to support your research.
Our intuitive apps will give you a better understanding of the core principles of electrochemistry, allowing you to correctly describe your reaction conditions and in turn bring greater success in creating you chemical products.
