Deeper analysis of electrochemical processes
If you want to dig deeper into the processes taking place in a particuar electrochemical reaction then cyclic voltammetry (CV) is an analytical tool that is used to measure the redox potentials for electrochemical processes. It requires additional equipment, and there are some really useful pointers in the linked article at the bottom of this page.
The redox potential is useful, as this helps interpret the electrochemical reactions and ultimately allows the electrosynthetic chemist (you!) to control an electrochemical transformation through modifying the energy of the electrons in the electrode. CV can be used to help understand reaction mechanisms and the reversibility of a reaction. In addition to the working electrode and counter electrode that are required in a synthetic setup, a further electrode – the reference electrode – is required for CV. The ElectroReact contains additional ports in the cap into which a reference electrode can be readily inserted.
Using an automated potentiostat, the potential between the working electrode and the reference electrode can then be controlled and the current measured. Typically a sweep over a range of potentials is carried out, and at each point the current recorded. The potential at which the maximum current is observed is the redox potential of the chemical transformation being studied and the points where solvent reduction and oxidation can often be identified, helping define a practical working range.
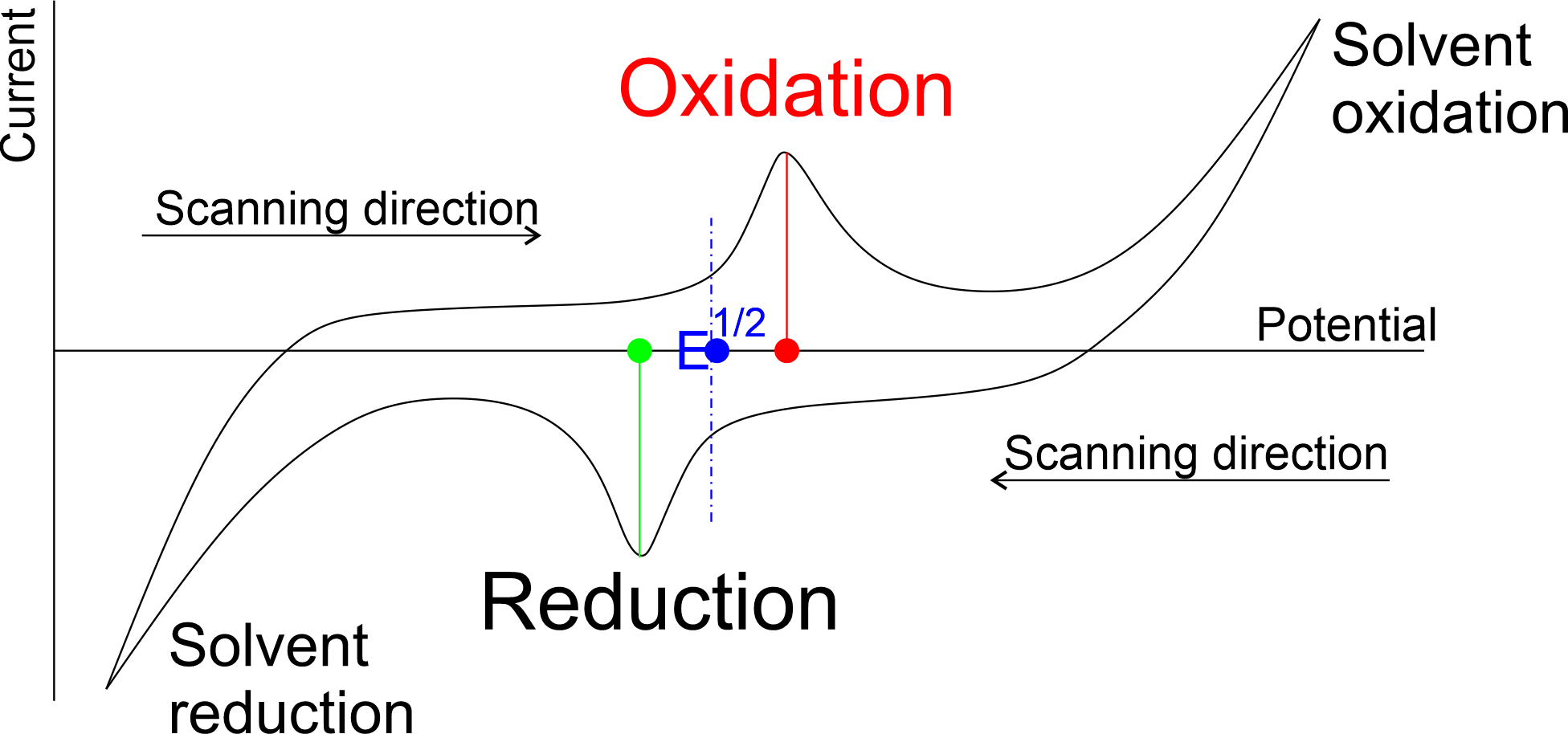
As the measured potential is dependent upon several factors, such as the reference electrode used, electrolyte concentration and solvent, it is important to report the full set of conditions under which this is measured. An internal standard with a well-known redox system, such as ferrocene/ferrocenium, may also be added to the system and the potential referenced against this. A reference electrode is used as a reference point against which the potential of the working electrode is measured. Commonly used electrodes in aqueous media include AgCl/Ag, standard hydrogen electrode (SHE) and saturated calomel electrode (SCE). In organic media, a silver wire in a solution containing a Ag+ salt (usually AgNO3) is used.
A useful guide that introduces the beginner to practical cyclic voltammetry has been written by Jillian Dempsey and co-workers:J. Chem. Educ. 2018, 95, 2, 197–206.
